It refers to what energy level it is and will be one greater than the number of nodes in the orbital. Therefore L is 6 and the magnetic quantum numbers are -6-5-4-3-2-10123456.

Quantum Theory Review 1 Zinc 30 Would Have Its Final Electrons Placed In What Sublevel 1 S 2 P 3 D 4 F Table Ppt Download
N is the first quantum number.
. What are the possible values for the first quantum number. It is the principle quantum number. What is the second quantum number of a 1s2 electron in phosphorus 1s22s22p63s23p3.
What does the second quantum number l describe. L is the second quantum number. What spin a specific.
What does the second quantum number describe. It refers to what energy level it is and will be one greater than the number of nodes in the. The second quantum number or l which describes the subshell s p d f etc.
The specific orbital within a sublevel. What does the second quantum number 1 describe. Chemistry 18032021 0310 kbuhvu.
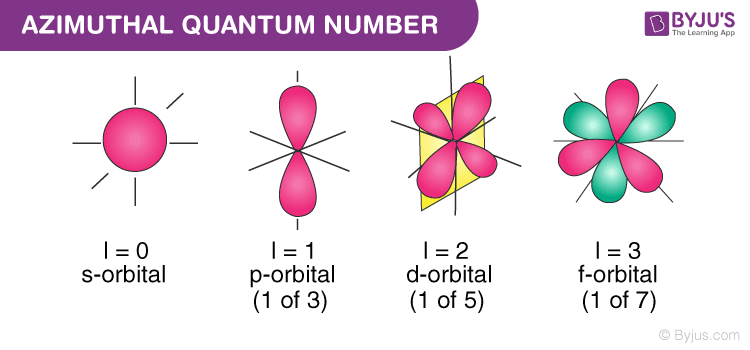
SECONDARY QUANTUM NUMBER l - Represents the energy sublevel or type of orbital occupied by the electron. This number is sometimes also called azimuthal or subsidiary. What does the third quantum number describe.
Not Just for Electrons While quantum numbers are commonly used to describe electrons they may be used to describe the nucleons protons and neutrons of an atom or elementary particles. It refers to what energy level it is and will be onegreater than the number of nodes in the orbital. Direction of spin of electrons.
It is the principle quantumnumber. The specific orbital within a sublevel. L is the secondquantum number.
Quantum numbers are a set of variables which describe pertinent characteristics of electrons within an atom. The value of l depends on the value of n such that l 0 1. What does the third quantum number m1 describe.
The four quantum numbers used to describe the electrons are n2 ℓ1 m1 0 or -1 and s12 the electrons have parallel spins. What does the second quantum number 1 describe. Shape of electron cloud.
Which sublevel the electron is in O C. Magnetic quantum number is limited by the azimuthal quantum number which is the quantum number describing the possible shapes. In chemistry and spectroscopy ℓ 0 is called an s orbital ℓ.
N is the principal quantum number which is 7. N principle quantum number 2 l angular momentum quantum number 0 ml magnetic quantum number 0 ms spin quantum number 12 or -12 Possible values of the magnetic quantum number m. It is the angular momentum.
What does the fourth quantum number describe. What does the second quantum number 1 describe. What spin a specific electron has B.
The azimuthal is given as L n-1. Which energy level is being occupied C. The second quantum number known as the angular or orbital quantum number describes the subshell and gives the magnitude of the orbital angular momentum through the relation.
The second quantum number or l which describes the subshell s p d f etc. What does the second quantum number 1 describe. What do quantum numbers describe.
Which energy level is being occupied O D. Which sublevel the electron is in B.

Azimuthal Quantum Number Definition Subsidiary And Angular Momentum Quantum Numbers

Quantum Numbers Free Textbooks Khan Academy Never Stop Learning

0 Comments